| cr_home | Metalle | Strukturchemie | Interm. Phasen | Oxide | FK-Chemie | Silicate | Strukturtypen |
| Inhalt | 1. Einleitung | 2. Wasserstoff | 3. Edelgase | 4. Halogene | 5. Chalkogene | 6. Pentele | 7. Tetrele | 8. Bor |
|
Vorlesung Chemie der Nichtmetalle6. Pnicogene (Pentele): N, P, As6.5. Sauerstoffsäuren |
 |
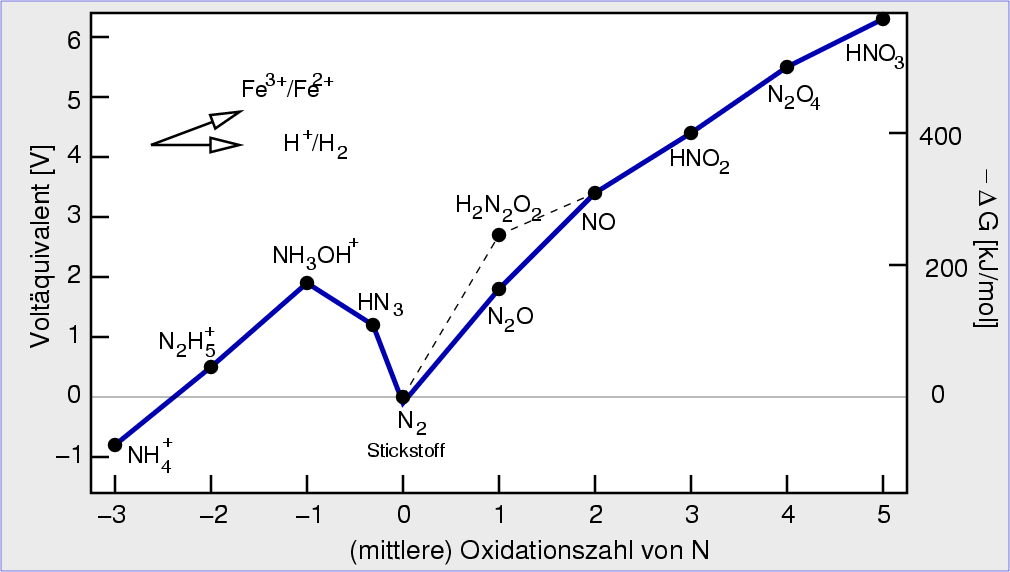
| Abb. 6.5.1. Frostdiagramm von N-O-Spezies ‣SVG |
| Allgemeine Formel | OS | (1) H3POn, m=1 | (2) H4P2On, m=2 | (3) Kondensation (...Oligo-.. Poly-Säuren) | (4) Meta-S. | |||||
| Mono-Säuren | Di-Säuren | m=∞ | ||||||||
| n | Formel | Säure (Salze) | n | Formel | Säure (Salze) | Formel | Säure (Salze) | |||
| +1 | 2 | H3PO2 | Phosphin-S. (Phosphinate) | |||||||
| +2 | 4 | H4P2O4 | Hypodiphosphon-S. (Hypodiphosphonate) | |||||||
| Hm+2PmO2m+1 | +3 | 3 | H3PO3 | Phosphon-S. (Phosphonate) | 5 | H4P2O5 | Diphosphon-S. (Diphosphonate) | ... | HPO2 | Metaphosphorige S. |
| Hm+2PmO2m+2 | +4 | 6 | H4P2O6 | Hypodiphosphor-S. (Hypodiphosphate) | ||||||
| Hm+2PmO3m+1 | +5 | 4 | H3PO4 | Phosphor-S. (Phosphate) | 7 | H4P2O7 | Diphosphor-S. (Diphosphate) | ... | HPO3 | Metaphosphor-S. (Metaphosphate) |
| Peroxo- Verbindungen | +5 | 8 | H4P2O8 | Peroxodiphosphor-S. (Peroxodiphosphate) | ||||||
| 5 | H3PO5 | Peroxophosphor-S. (Peroxophosphate) | ||||||||
| 6 | H3PO6 | Diperoxophosphor-S. (Diperoxophosphate) | ||||||||
 |
| Abb. 6.5.2. Lagediagramm P-O-H ‣SVG |
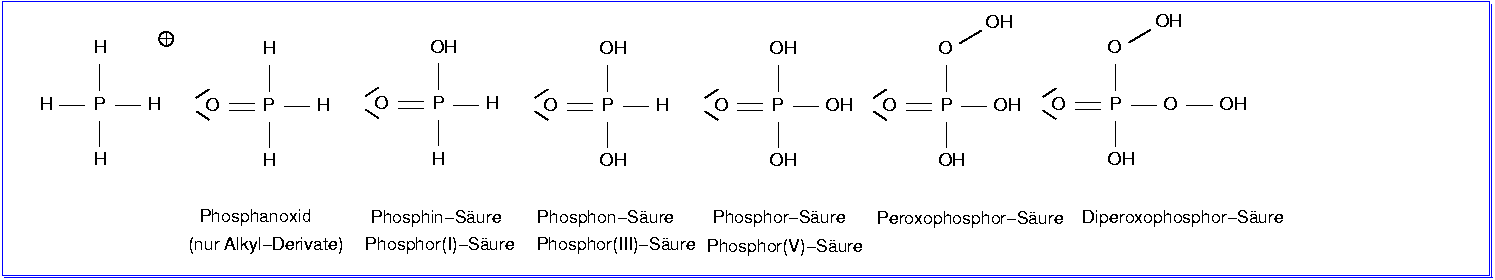 |
| Abb. 6.5.2. Mono-Säuren ‣SVG |
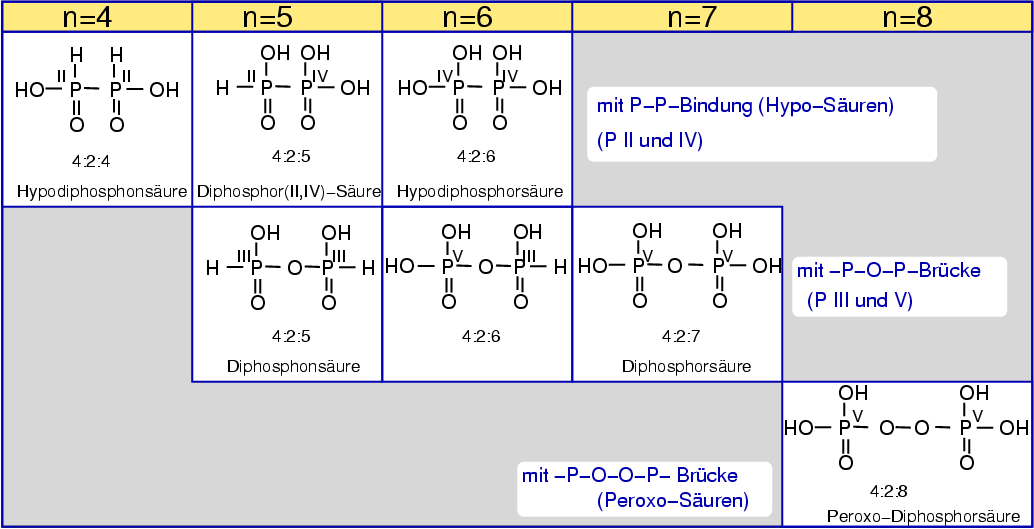 |
| Abb. 6.5.3. Di-Säuren ‣SVG |
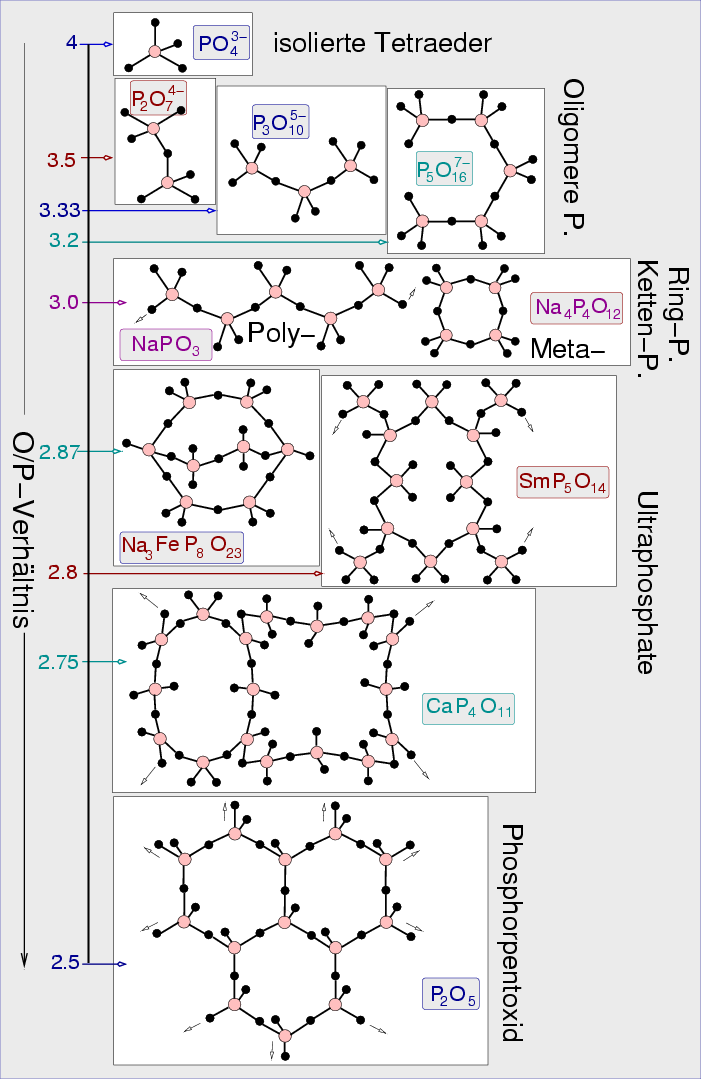 |
| Abb. 6.5.4. Strukturelemente in Phosphaten ‣SVG |
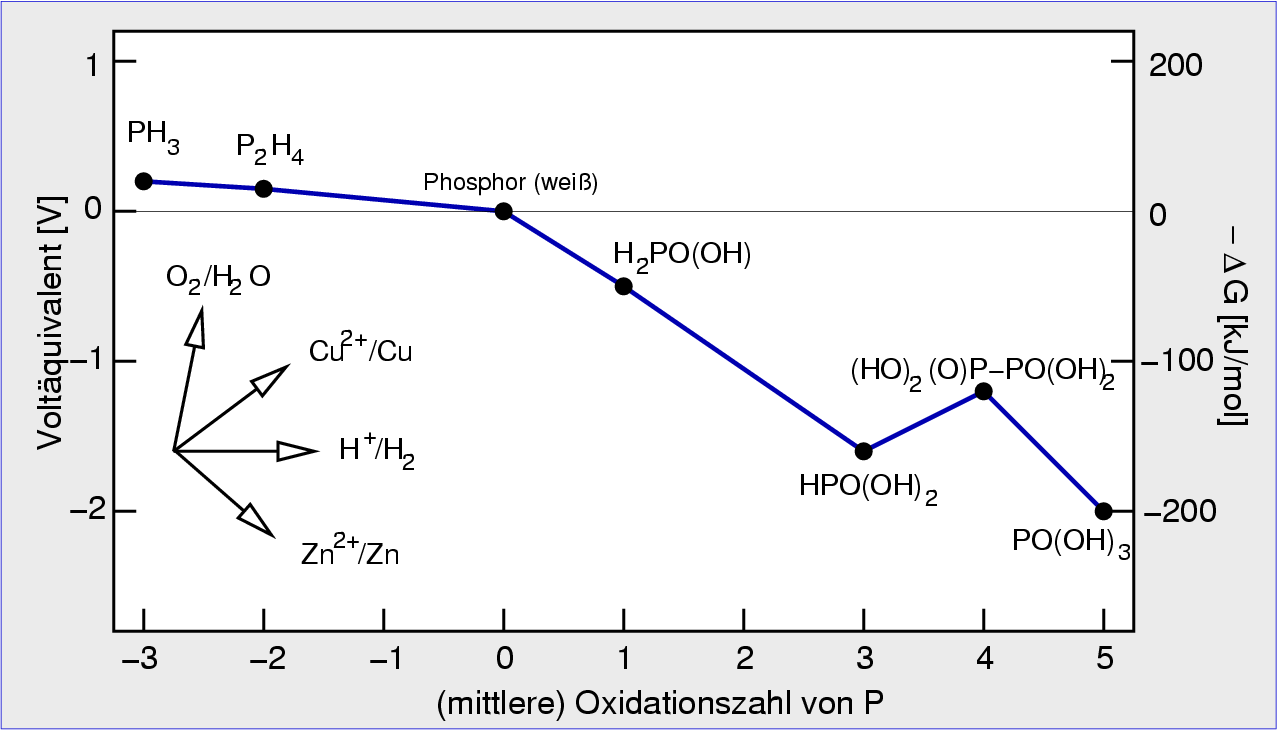 |
| Abb. 6.5.5. Frostdiagramm von P-O-Spezies ‣SVG |
| Inhalt | 1. Einleitung | 2. Wasserstoff | 3. Edelgase | 4. Halogene | 5. Chalkogene | 6. Pentele | 7. Tetrele | 8. Bor |
|
| cr_home | Metalle | Strukturchemie | Interm. Phasen | Oxide | FK-Chemie | Silicate | Strukturtypen |